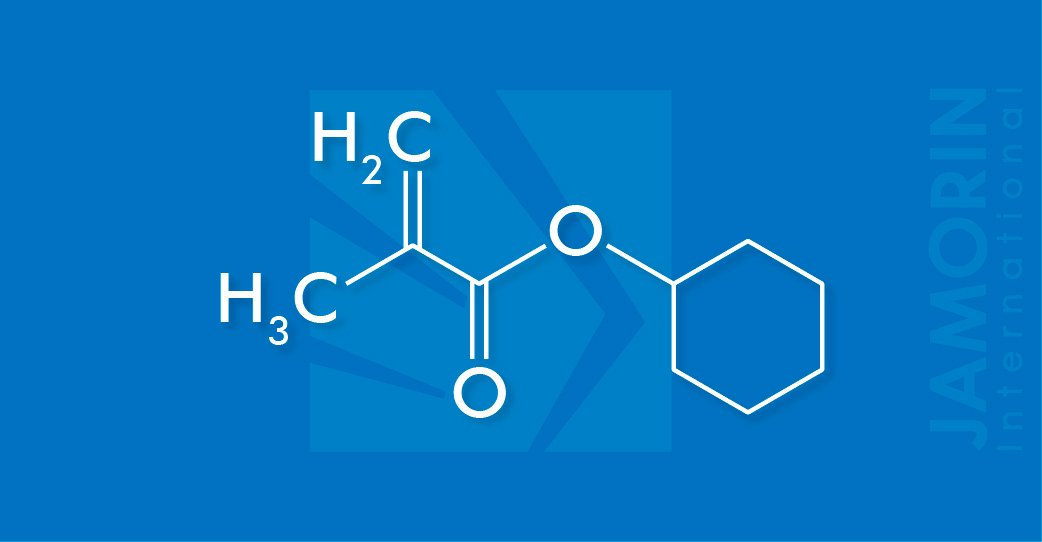
C10 H16 O2
min. 98.0 %
C10 H16 O2
min. 98.0 %
Cyclohexyl Methacrylate (CHMA)
Cyclohexyl Methacrylate (CHMA) is an ester of Methacrylic acid and is used as a raw material component in the synthesis of polymers. Cyclohexyl Methacrylate (CHMA) is a monofunctional monomer consisting of a methacrylate group with a characteristic high reactivity and a cyclic hydrophobic group. Copolymers of Cyclohexyl Methacrylate (CHMA) can be prepared with (meth)acrylic acid and its salts, amides, and esters, and with (meth)acrylates, acrylonitrile, maleic acid esters, vinyl acetate, vinyl chloride, vinylidene chloride, styrene, butadiene, unsaturated polyesters and drying oils, etc.
Cyclohexyl Methacrylate (CHMA) is a very useful starting material for chemical synthesis since it easily undergoes addition reactions with a wide range of organic and inorganic compounds.
Product type: Methacrylate Monomer
Features & Benefits:
- Chemical resistance
- Hydrophobicity
- Hydrolytic stability
- Scratch-resistant
- Adhesion
- Weather resistance
It is applied in the production of:
- Coatings
- Personal care
- Adhesives
Chemical Properties:
-
Puritymin. 98.0 %
-
Acid Valuemax. 0.01 % (as methacrylic acid)
-
Water contentmax. 0.1 %
-
Color APHAmax. 100
Physical Properties:
-
AppearanceClear, colorless
-
Physical formliquid
-
Odorester-like
-
Molecular weight168.2 g/mol
-
Polymer Tg105 °C
-
Tg80 °C
-
Density0.961 / 0.9374 g/cm3 at 20 / 50 °C
-
Boiling Point93.75 °C at 20 mbar
-
Flash point81 °C
-
Melting Point–104 °C
-
Viscosity2.32 mPa · s at 23.3 °C
-
Vapor Point0.184 mbar at 20 °C
In order to prevent polymerisation, Cyclohexyl Methacrylate (CHMA) must always be stored under air, and never under inert gases. The presence of oxygen is required for the stabiliser to function effectively. It has to contain a stabiliser and the storage temperature must not exceed 35 °C. Under these conditions, the storage stability of one year can be expected upon delivery. In order to minimize the likelihood of over storage, the storage procedure should strictly follow the “first-in-first-out” principle. For extended storage periods over 4 weeks, it is advisable to replenish the dissolved oxygen content. If Cyclohexyl Methacrylate (CHMA) is crystallized the product should never be partially molten and taken because of the possible separation from the stabiliser. Ensure that there is no crystallised product in the container before use. Obtain information from supplier/manufacturer before dissolving totally or partially crystallised products. The ambient temperature of the container may not exceed the stated temperature limit when melting the product or keeping it at a moderate temperature.The preferred construction material for tanks and pipes is stainless steel. Carbon steel is also acceptable, although the formation of rust may be a problem with product quality (color). Iron(III)-ions have been shown to be a weak polymerisation initiator. If carbon steel is to be used, special procedures should be used to prepare the tank for use. Storage tanks, pumps, and pipes should be earthed.
Storage & Handling:
In order to prevent polymerisation, Cyclohexyl Methacrylate (CHMA) must always be stored under air, and never under inert gases. The presence of oxygen is required for the stabiliser to function effectively. It has to contain a stabiliser and the storage temperature must not exceed 35 °C. Under these conditions, the storage stability of one year can be expected upon delivery. In order to minimize the likelihood of over storage, the storage procedure should strictly follow the “first-in-first-out” principle. For extended storage periods over 4 weeks, it is advisable to replenish the dissolved oxygen content. If Cyclohexyl Methacrylate (CHMA) is crystallized the product should never be partially molten and taken because of the possible separation from the stabiliser. Ensure that there is no crystallised product in the container before use. Obtain information from supplier/manufacturer before dissolving totally or partially crystallised products. The ambient temperature of the container may not exceed the stated temperature limit when melting the product or keeping it at a moderate temperature.The preferred construction material for tanks and pipes is stainless steel. Carbon steel is also acceptable, although the formation of rust may be a problem with product quality (color). Iron(III)-ions have been shown to be a weak polymerisation initiator. If carbon steel is to be used, special procedures should be used to prepare the tank for use. Storage tanks, pumps, and pipes should be earthed.
A Safety Data Sheet has been compiled for Cyclohexyl Methacrylate (CHMA) that contains up-to-date information on questions relevant to safety.
The data contained in this publication are based on our current knowledge and experience. In view of the many factors that may affect the processing and application of our product, these data do not relieve processors from carrying out their own investigations and tests; neither do these data imply any guarantee of certain properties, nor the suitability of the product for a specific purpose. Any descriptions, drawings, photographs, data, proportions, weights, etc. given herein may change without prior information and do not constitute the agreed contractual quality of the product. It is the responsibility of the recipient of our products to ensure that any proprietary rights and existing laws and legislation are observed.